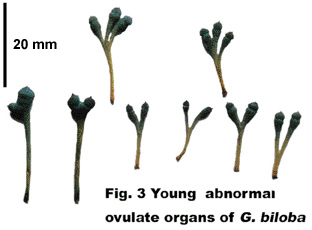
Ginkgo biloba is a long-lived, dioecious and deciduous tree up to more than 30 meters high and 10 meters in girth (Figure 1). It bears flabellate leaves with a long petiole and dichotomously branching veins, which grow either in tuft at the terminal end of dwarf shoots or are attached sparsely to the newly-sprung long shoot (Figures 2-3). It has a long fossil record and is well known as having the preeminent claim to be described as a living fossil (Seward, 1911, 1919). Living Ginkgo is called “Baiguo” (white nut), “Yajiao” (duck foot), “Gongsunshu” (grandfather/grandson tree), “Yinxing” or “Icho” (silver apricot) and “maidenhair tree” in China, Japan and other countries. However, the name Ginkgo originates from 1712 when the traveller Kaempfer proposed for this plant. Linnaeus, the founder of the binomial system of nomenclature, adopted this designation adding the species name ‘biloba‘ to denote the bisection of the wedge-shaped lamina of the leaf into two divergent segments (Seward, 1911). According to Tori and Tori (1997), the name Ginkgo was derived from “Ginkyo” which is a Japanese pronounciation of the Chinese characters “Yinxing”.


In the modern day flora Ginkgo biloba is the sole representative of a once greatly flourished plant group in geological history – Ginkgoales. For a long time, our knowledge of the evolutionary history of Ginkgo and its allies was based mainly on leaf fossils (Seward, 1911; Florin, 1936; Tralau, 1967, 1968; Harris et al., 1974). Recent studies on fossil reproductive organs revealed that vegetative leaves previously referred to Ginkgo, or ginkgophytes are rather heterogeneous (Zhou, 1991, 1997). Quite a number of so-called ginkgophyte fossils belong to other extinct plant groups than Ginkgoales, such as Czekanowskiales which bear characteristic bivalve capsules containing rows of seeds are markedly distinguished from Ginkgo (Harris, 1951; Krassilov, 1970).
Different hypotheses have been proposed for the origin and relationships of Ginkgoales by palaeobotanists and botanists based on molecular biological as well as comparative morphological approaches (Zimmermann, 1959; Crane, 1985; Doyle and Donoughue, 1986; Meyen, 1987; Archangelsky and Cueneo, 1990; Zhou, 1991; Stewart and Rothwell, 1993; Chase et al., 1993; Rothwell and Serbet, 1994; Haseba, 1997; Burleigh and Mathews, 2004). There is yet no direct fossil evidence and unanimously accepted theory about the origin of ginkgoales, although most believe that they were originated from progymnosperms, related either to pteridosperms and cycads, or to cordaites and conifers (Kenrick and Crane, 1997). Their precise ancestry is therefore uncertain.

The earliest representative of Ginkgoales that exhibits the essential characters of Ginkgo can be traced back to the early Permian. Trichopitys Saporta (Florin, 1949) from the Autunian (Permian) in Lodéve, southern France bears vegetative shoots with spirally arranged, not flattened dichotomously branching leaves and axillary fertile shoots with up to 20 pedicellate ovules on the distal end of lateral branches (Figure 4). Some authors, however, consider Trichopitys to be related to peltaspermalean pteridosperms Dichophyllum and Bairmopteris (Meyen, 1987), or the enigmateic seed plants Polyspermophyllum and Dicranophyllum (Archangelsky and Cueneo, 1990). In the late Permian, flattened and laminated leaves of the Sphenobaiera type occurred, but no petiolate ginkgoalean leaves existed yet.
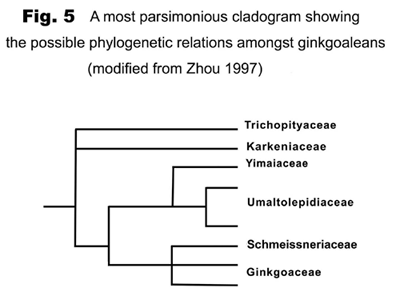
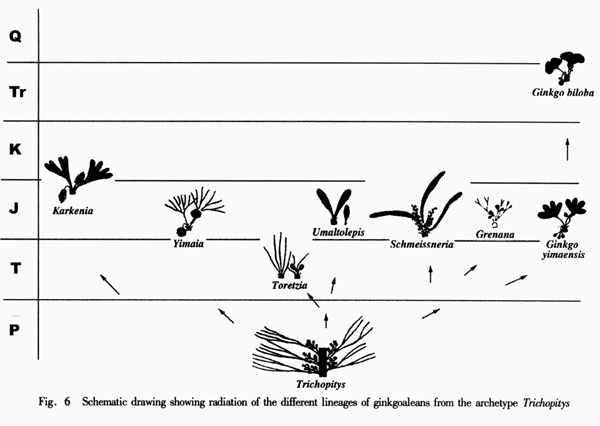
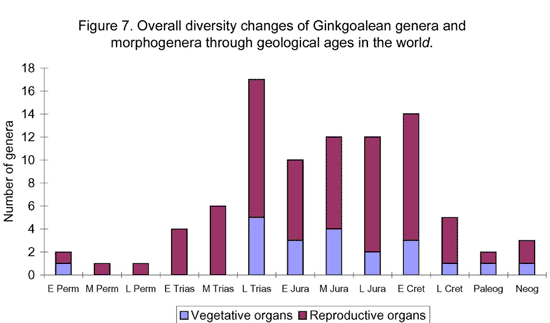
Ginkgoaleans drastically diverge and radiate in the early Mesozoic. In the late Triassic, almost all known evolutionary lineages (families) were simultaneously present (Figures 5-6). The diversity of both vegetative and reproductive organs reached the highest peak in the geological history respectively (Figure 7). The emergence of a large number of new taxa, the rapid increase in geographical distribution, and the simultaneous accomplishment of major morphological innovations also supports an early Mesozoic, notably late Triassic radiation of ginkgoaleans (Zhou and Wu, 2006).
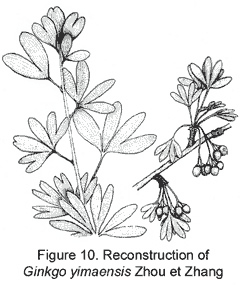
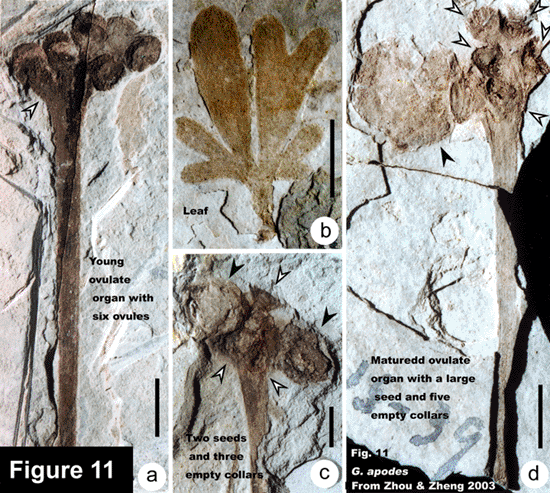
The representative members of the five lineages so far known in the Mesozoic ginkgoaleans are as follows: Ginkgo (Ginkgoaceae including Grenana Samylina 1991 and possibly Nehvizdyella Kvacek et al. 2005), Karkenia (Karkeniaceae) (Archangelsky, 1965), Toretzia and Umaltolepis (Umaltolepidiaceae) (Stanislavsky, 1973; Krassilov, 1970, 1972), Yimaia (Yimaiaceae) (Zhou and Zhang, 1992) (Figure 8) and Schmeissneria (Schmeissneriaceae) (Kirchner and Van Konijnenburg-van Cittert, 1994). Authentic pollen organs are hitherto only scarcely known (Van Konijnenburg-van Cittert, 1971). Ginkgoalean vegetative organs, especially leaves are abundant in the Mesozoic strata. The leaves are of considerably varied shape and extent of division even within a single species. On the other hand, similar-looking vegetative organs may belong to different mother plants. For instance, Ginkgoites type leaves are known in different genera belonging respectively to three families (Zhou, 1997).

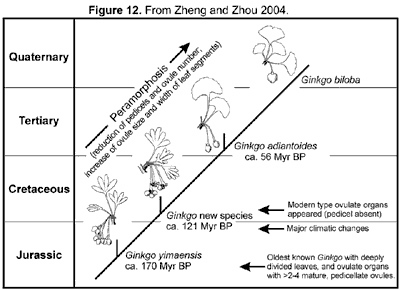
As compared with the late Palaeozoic Trichopitys, all the Mesozoic members have reduced (shortened) shoots (dwarf shoots). Their leaves tend to be laminated, and petiolate (becoming complete), and the deeply divided laminae tend to be fused and webbed. The ovulate organs are generally markedly reduced. Except for those in Karkeniaceae, ovules decrease in number and increase in size, and pedicels become shortened and eventually lost. The general evolutionary trend of ginkgoaleans is reduction of both reproductive and vegetative organs (Zhou, 1991, 1997).

Ginkgoaleans were greatly flourished and had a world-wide distribution during the great part of Mesozoic. From the late Cretaceous onward, ginkgoaleans rapidly decreased. In the late Cretaceous and early Tertiary, Ginkgo had a circum-polar distribution, but retreated from North America in the late Miocene (about 10 Myr ago) and became extinct in Europe in Pliocene (about 17 Myr ago) (Wolf, 1987; Kovar-Eder et al., 1994; Samylina, 1967). In East Asia, the latest Ginkgo fossils have been recoded from the late Pliocene and Pleistocene in Japan (Uemura, 1997). In China, there is no record of Ginkgo in sediments younger than Eocene. Whether Ginkgo is a refugee that has existed during the whole Cenozoic there or just an immigrant from Japan to the Yangtze valley quite recently remains unknown.
Contribution by Professor Zhiyan Zhou
Cited literature:
Archangelsky SA. 1965. Fossil Ginkgoales from the Ticò flora, Santa Cruz Province, Argentina. Bulletin of the British Museum (Natural History) Geolology 10: 121-137.
Archangelsky SA, Cueneo R. 1990. Polyspermophyllum, a new Permian gymnosperm from Argentina. Review of Palaeobotany and Palynology 63: 117-135.
Burleigh JG and S Mathews, 2004. Phylogenetic signal in nucleotide data from seed plants: implications for resolving the seed plant tree of life. American Journal of Botany 91: 1599-1613.
Chase MW, Soltis DE, Olmstead RG et al., 1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528-580.
Crane PR 1985. Phylogenetic analyses of seed plants and the origin of angiosperms. Annals of the Missouri Botanical Garden 72: 716-793.
Crane PR, Manchester SR, Dilcher DL. 1990. A preliminary survey of fossil leaves and well-preserved reproductive structures from the Sentinel Butte formation (Paleocene) near Almont, North Dakota. Fieldiana: Geology (New series) 20: 1-63.
Doyle JA, Donoughue MJ. 1986. Seed plant phylogeney and the origin of angiosperms: an experimental cladistic approach. Botanical Reviews 52: 321-431.
Florin R. 1936a, b. Die fossilen Ginkgophyten von Franz-Joseph-Land nebst Erörterungen über vermeintliche Cordaitales mesozoischen Alters I, II. Palaeontographica (B) 81: 71-173; 82: 1-72.
Florin R.1949. The morphology of Trichopitys heteromorpha Saporta, a seed plant of Palaeozoic age, and the evolution of female flowers in the Ginkgoinae. Acta Horti Bergiana 15(5):158-182.
Harris TM. 1951. The fructification of Czekanowskia and its allies. Philosophical Transactions of the Royal Society London (B) 235: 483-508
Harris TM, Millington W, Miller J. 1974. The Yorkshire Jurassic flora IV. British Museum (Natural History): London.
Hasebe M. 1997. Molecular phylogeney of Ginkgo biloba: close relation between Ginkgo biloba and cycads. In Ginkgo biloba – A Global Treasure from Biology to Medicine (Editors Hori T, Ridge RW, Tulecke W, Del Tredici P, Trémouillaux-Guiller J, and Tobe H). Springer Verlag: Tokyo, 173-181.
Kenrick P, Crane PR. 1997. The origin and early diversification of land Plants: a cladistic study. Smithsonian Institution Press: Washington and London.
Kirchner M, Van Konijnenburg-van Cittert JHA. 1994. Schmeissneria microstachys (Presl, 1833) Kirchner et Van Konijnenburg-van Cittert, gen. et sp. nov., plants with ginkgoalean affinities of Germany. Review of Palaeobotany and Palynology 83: 199-215.
Kovar-Eder J, Givulescu L, Hably L et al. 1994. Floristic changes in the areas surrounding the Paratethys during Neogene time. In Boulter MC, Fisher HC. Cenozoic plants and climate of the Arctic. Springer Verlag: Berlin, 347-369.
Krassilov VA. 1970. An approach to the classification of Mesozoic ginkgoalean plants from Siberia. Palaeobotanist 18: 12-19.
Krassilov VA. 1972. Mesozoic flora from the Bureja river (Ginkgoales and Czekanowskiales). Nauka: Moscow.
Kvacek J, Falcon-Lang L, Dašková J. 2005. A new late Cretaceous ginkgoalean reproductive structure Nehvizdyella gen, nov. from the Czech Republic and its whole-plant reconstruction. American Journal of Botany 92: 1958-1969.
Meyen SV. 1987. Fundamentals of Palaeobotany. Chapman & Hall: London & New York.
Rothwell GW, Serbet R. 1994. Lignophyte phylogeney and the evolution of spermatophytes: a numerical cladistic analysis. Systematic Botany 19: 443-482.
Samylina VA. 1967. On the final stage of the history of the genus Ginkgo L. in Eurasia. Bot. Zhurn 52: 303-316 (in Russian).
Seward AC. 1911. Links with the past in the plant world. Cambridge University Press: Cambridge.
Seward AC. 1919. Fossil Plants IV. Cambridge University Press: Cambridge.
Stanislavsky FA. 1973. The new genus Toretzia from the Upper Triassic of the Donetz basin and its relation to the genera of the order Ginkgoales. Paleont Zhurn (1): 88-96 (in Russian).
Stewart WN, Rothwell GW. 1993. The Biology and Evolution of Plants. Cambridge University Press: Cambridge.
Tralau H. 1967. The phytogeographic evolution of the geneus Ginkgo L. Bot. Notis 120: 409-422.
Tralau H. 1968. Evolutionary trends in the genus Ginkgo. Lethaia 1: 63-101.
Uemura K. 1997. Cenozoic history of Ginkgo in East Asia. In Ginkgo biloba – A Global Treasure from Biology to Medicine (Editors Hori T, Ridge RW, Tulecke W, Del Tredici P, Trémouillaux-Guiller J & Tobe H). Springer Verlag: Tokyo; 207-221.
Van Konijnenburg-van Cittert JHA. 1971. In situ gymnosperm fructifications from the Jurassic flora of Yorkshire. Acta Bot Neerl 20:1-96.
Wolf JA. 1987. An overview of the origins of modern vegetation and flora of the northern Rocky Mountain. Annals of the Missouri Botanical Garden, 74;785-803.
Zheng SL, Zhou ZY. 2004. A new Mesozoic Ginkgo from western Liaoning, China and its evolutionary significance. Review of Palaeobotany and Palynology 131: 91-103.
Zhou ZY, Wu XW, 2006. The rise of ginkgoalean plants in the early Mesozoic: a data analysis. Geological Journal 41: 363-375.
Zhou ZY, Zhang BL. 1989. A Middle Jurassic Ginkgo with ovule-bearing organs from Henan, China. Palaeontographica (B) 211: 113-133.
Zhou ZY, Zhang BL. 1992. Baiera hallei Sze and associated ovule-bearing organs from the Middle Jurassic of Henan, China. Palaeontographica (B) 224: 151-169.
Zhou ZY, Zheng SL. 2003. The missing link of Ginkgo evolution. Nature 423: 821-822.
Zhou ZY. 1991. Phylogeny and evolutionary trends of Mesozoic ginkgoaleans – a preliminary assessment. Review of Palaeobotany and Palynology 68: 203-216.
Zhou ZY. 1997. Mesozoic ginkgoalean megafossils: a systematic review. In Ginkgo biloba – A Global Treasure from Biology to Medicine (Editors Hori T, Ridge RW, Tulecke W, Del Tredici P, Trémouillaux-Guiller J & Tobe H). Springer Verlag: Tokyo; 183-206.
Zimmermann W. 1959. Die Phylogene der Pflanzen. Fisher Verlag: Stuttgart.
